
Curriculum Vitae
| Carrier | Latvian Institute of Organic Synthesis, Riga, Latvia Since 2019 Principal Researcher 2017 Researcher 2011 Research Assistant 2009 Laboratory Assistant |
| Education | 2017 Dr. Chem., Faculty of Materials Science and Applied Chemistry, Riga Technical University 2015 (Sept)–2016 (May) InnovaBalt Exchange Program, University of Perugia, Italy (Prof. L. Vaccaro Group) 2011 M.Sc. Chem., Faculty of Chemistry, University of Latvia 2009 B.Sc. Chem., Faculty of Chemistry, University of Latvia 2008 (Oct)–2009 (Feb) Erasmus Exchange Program, Leibniz University Hanover, Germany |
| Awards | 2016 Scientific Achievement of the Year by Latvian Academy of Science 2012 Latvian Academy of Science, Martins Straumanis–Alfreds Ievins Award for the Master Thesis 2011 LLC “Bapeks” Poster Award in 7th Paul Walden Symposium on Organic Chemistry 2005–2007 Zvejnieku Family Scholarship from Foundation “Vitols Fund” |
List of Publications
16. Semisynthesis of Linariophyllenes A–C and Rumphellolide H, Structure Revisions and Proposed Biosynthesis Pathways
Stakanovs, G.; Blazevica, A.; Belyakov, S.; Rasina, D.; Jirgensons, A. J. Nat. Prod., 2023, 86, 2368–2378. DOI:10.1021/acs.jnatprod.3c00574

15. Macrocyclic Peptidomimetic Plasmepsin X Inhibitors with Potent In Vitro and In Vivo Antimalarial Activity
Kovada, V.; Withers-Martinez, C.; Bobrovs, R.; Ce̅rule, H.; Liepins, E.; Grinberga, S.; Hackett, F.; Collins, C. R.; Kreicberga, A.; Jiménez-Díaz, M. B.; Angulo-Barturen, I.; Rasina, D.; Suna, E.; Jaudzems, K.; Blackman, M. J.; Jirgensons, A. J. Med. Chem. 2023, 66, 15, 10658–10680. DOI:10.1021/acs.jmedchem.3c00812

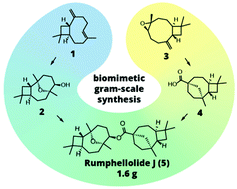
14. Convergent biomimetic semisynthesis of disesquiterpenoid rumphellolide J
Stakanovs, G.; Belyakov, S.; Jirgensons, A.; Rasina, D. Org. Biomol. Chem., 2022, 20, 2455-246. DOI:10.1039/D2OB00238H
13. Exploring Aspartic Protease Inhibitor Binding to Design Selective Antimalarials
Bobrovs, R.; Basens, E. E.; Drunka, L.; Kanepe, I.; Matisone, S.; Velins, K. K.; Andrianov, V.; Leitis, G.; Zelencova-Gopejenko, D.; Rasina, D.; Jirgensons, A.; Jaudzems, K. J. Chem. Inf. Model., 2022, 62, 3263–3273. DOI:10.1021/acs.jcim.2c00422

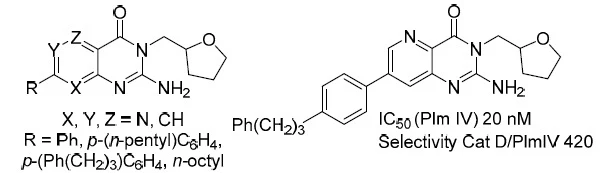
12. Synthesis of 2-Aminopyridopyrimidinones and Their Plasmepsin I, II, IV Inhibition Potency
Rasina, D.; Stakanovs, G.; Kanepe-Lapsa, I.; Bobrovs, R.; Jirgensons, A., Chem. Heterocycl. Compd., 2020, 56, 786-792. DOI:10.1007/s10593-020-02731-3
11. A Concise Bioinspired Semisynthesis of Rumphellaones A–C and Their C-8 Epimers from β-Caryophyllene
Stakanovs, G.; Mishnev, A.; Rasina, D.; Jirgensons, A., J. Nat. Prod. 2020, 83, 6, 2004-2009. DOI:10.1021/acs.jnatprod.0c00403

10. A Novel Collaborative Approach to Facilitate Chemical Biology
Brennecke, P.; Rasina, D.; Aubi, O.; Herzog, K.; Landskron, J.; Cautain, B.; Vicente, F.; Quintana, J.; Mestres, J.; Stechmann, B.; Ellinger, B.; Brea, J; Kolanowski, J. L.; Pilarski, R.; Orzaez, M.; Pineda-Lucena, A.; Laraia, L.; Nami, F.; Zielenkiewicz, P.; Paruch, K.; Hansen, E.; Von Kries, J. P.; Neuenschwander, M.; Specker, E.; Bartunek, P.; Simova, S.; Leśnikowski, Z.; Krauss, S.; Lehtiö, L.; Bilitewski, U.; Brönstrup, M.; Taskén, K.; Jirgensons, A.; Lickert, H.; Clausen, M. H.; Andersen, J. H.; Vicent, M. J.; Genilloud, O.; Martinez, A.; Nazaré, M.; Fecke, W.; Gribbon, P.
SLAS Discov., 2019, 24, 398–413. DOI:10.1177/2472555218816276

9. 2-Aminoquinazolin-4(3H)-one Based Plasmepsin Inhibitors with Improved Hydrophilicity and Selectivity
Rasina, D.; Stakanovs, G.; Borysov, O.V.; Pantelejevs, T.; Bobrovs, R.; Kanepe-Lapsa, I.; Tars, K.; Jaudzems, K.; Jirgensons, A. Biorg. Med. Chem., 2018, 26(9), 2488-2500. DOI:10.1016/j.bmc.2018.04.012

8. N-Sulfonylcarboxamide as an Oxidizing Directing Group for Ruthenium-Catalyzed C–H Activation/Annulation
Petrova, E.; Rasina, D.; Jirgensons, A. Eur. J. Org. Chem., 2017, 2017, 1773-1779. DOI:10.1002/ejoc.201601582

7. Targeting Multiple Aminoacyl-tRNA Synthetases Overcomes the Resistance Liabilities Associated with Antibacterial Inhibitors Acting on a Single Such Enzyme
Randall, C.; Rasina, D.; Jirgensons, A.; O’Neill, A. Antimicrob. Agents Chemother., 2016, 60(10) 6359-6361. DOI:10.1128/AAC.00674-16

6. A Polymorphism in LeuS Confers Reduced Susceptibility to GSK2251052 in a Clinical Isolate of Staphylococcus Aureus
Gupta, A.; Monteferrante, C.; Rasina, D.; Leitis, G.; Randall, C.P.; Tomlinson, J.H. Jirgensons, A.; Goessens, W.H.F.; Hays, J.P.; O’Neill, A.J. Antimicrob. Agents Chemother., 2016, 60(5), 3219-3221. DOI:10.1128/AAC.02940-15

5. Fragment-Based Discovery of 2-Aminoquinazolin-4(3H)-ones as Novel Class Nonpeptidomimetic Inhibitors of the Plasmepsins I, II, and IV
Rasina, D.; Otikovs, M.; Leitans, J.; Recacha, R.; Borysov, O.V.; Kanepe-Lapsa, I.; Domraceva, I.; Pantelejevs, T.; Tars, K.; Blackman, M.J.; Jaudzems, K.; Jirgensons, A. J. Med. Chem., 2016, 59, 374–387. DOI:10.1021/acs.jmedchem.5b01558

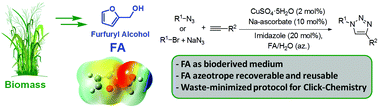
4. Searching for Novel Reusable Biomass-Derived Solvents: Furfuryl Alcohol/Water Azeotrope as a Medium for Waste-Minimised Copper-Catalysed Azide–Alkyne Cycloaddition
Rasina D.; Francesco F.; Santoro S.; Lombi A.; Vaccaro L. Green Chem., 2016, 18, 6380-6386. DOI:10.1039/C6GC01941B
3. Heterogeneous Palladium-Catalysed Catellani Reaction in Biomass-Derived γ-Valerolactone
Rasina D.; Kahler-Quesada A.; Ziarelli S.; Warratz S.; Cao H.; Santoro S.; Ackermann L.; Vaccaro L. Green Chem., 2016, 18, 5025-5030. DOI:10.1039/C6GC01393G

2. C-H Arylations of 1,2,3-Triazoles by Reusable Heterogeneous Palladium Catalysts in Biomass-Derived γ-Valerolactone
Tian X.; Yang F., Rasina D.; Bauer M.; Warratz S.; Ferlin F.; Vaccaro L.; Ackermann L. Chem. Commun. 2016, 52, 9777-9780. DOI:10.1039/C6CC03468C

1. 2-Vinyl Threoninol Derivatives via Acid-Catalyzed Allylic Substitution of Bisimidates
Kumar, V.; Klimovica, K.; Rasina, D.; Jirgensons A. J. Org. Chem., 2015, 80, 5934–5943. DOI:10.1021/acs.joc.5b00529
