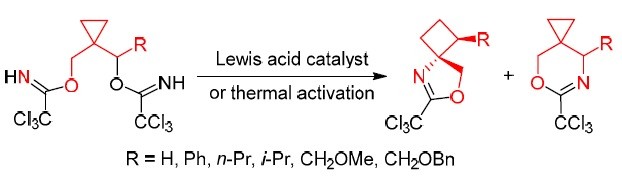
104. Electrochemical Formation of Oxazolines by 1,3-Oxyfluorination of Non-activated Cyclopropanes
Darzina, M.; Jirgensons, A. Org. Lett., 2024, asap. DOI:10.1021/acs.orglett.4c00143

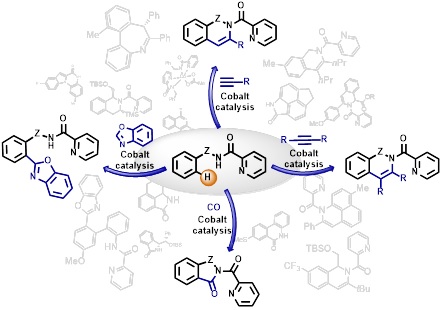
103. Indole Synthesis by Cobalt-Catalyzed Intramolecular Amidation via the Oxidatively Induced Reductive Elimination Pathway
Cizikovs, A.; Basens, E. E.; Zagorska, P. A., Kinens, A. Grigorjeva, L. ACS Catal., 2024, 14, 1690–1698. DOI:10.1021/acscatal.3c05706

102. Discovery of Malarial Threonyl tRNA Synthetase Inhibitors by Screening of a Focused Fragment Library
Bolsakova, J.; Bobrovs, R.; Varacheva, L.; Rudnickiha, A.; Kanepe, I.; Parisini, E.; Jirgensons, A. ACS Med. Chem. Lett. 2023, asap. DOI:10.1021/acsmedchemlett.3c00403

101. Exploring the Binding Pathway of Novel Nonpeptidomimetic Plasmepsin V Inhibitors
Bobrovs, R.; Drunka, L.; Kanepe, I.; Jirgensons, A.; Caflish, A.; Salvalaglio, M.; Jaudzems, K. J. Chem. Inf. Model. 2023, 63, 6890–6899. DOI:10.1021/acs.jcim.3c00826

100. Effect of novel furan-based ester reactive diluent on structure and properties of UV-crosslinked acrylated rapeseed oil
Briede, S.; Platnieks, O.; Darzina, M.; Jirgensons, A.; Gaidukovs, S. J. Polym. Sci., 2023, 1. DOI:10.1002/pol.20230451

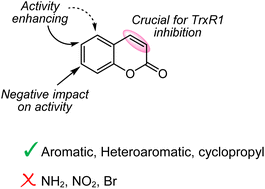
99. Design, Synthesis, and Bioactivity Evaluations of 3-Methylenechroman-2-one Derivatives as Thioredoxin Reductase (TrxR) Inhibitors
Nikitjuka, A.; Ozola, M.; Krims-Davis, K.; Žalubovskis, R. ChemMedChem, 2023, e202300504. DOI:10.1002/cmdc.202300504

98. Boron-containing carbonic anhydrases inhibitors
Giovannuzzi, S.; Nikitjuka, A.; Pereira Resende, B. R.; Smietana, M.; Nocentini, A.; Supuran, C. T.; Winum, J.-Y. Bioorg. Chem., 2024, 143, 106976. DOI:10.1016/j.bioorg.2023.106976

97. Exploration of 3,4-unsubstituted coumarins as thioredoxin reductase 1 inhibitors for cancer therapy
Nikitjuka, A.; Ozola, M.; Jackevica, L.; Bobrovs, R.; Žalubovskis, R. Org. Biomol. Chem., 2023, 21, 9630-9639. DOI:10.1039/D3OB01522J
96. Semisynthesis of Linariophyllenes A–C and Rumphellolide H, Structure Revisions and Proposed Biosynthesis Pathways
Stakanovs, G.; Blazevica, A.; Belyakov, S.; Rasina, D.; Jirgensons, A. J. Nat. Prod., 2023, 86, 2368–2378. DOI:10.1021/acs.jnatprod.3c00574

95. Macrocyclic Peptidomimetic Plasmepsin X Inhibitors with Potent In Vitro and In Vivo Antimalarial Activity
Kovada, V.; Withers-Martinez, C.; Bobrovs, R.; Ce̅rule, H.; Liepins, E.; Grinberga, S.; Hackett, F.; Collins, C. R.; Kreicberga, A.; Jiménez-Díaz, M. B.; Angulo-Barturen, I.; Rasina, D.; Suna, E.; Jaudzems, K.; Blackman, M. J.; Jirgensons, A. J. Med. Chem. 2023, 66, 15, 10658–10680. DOI:10.1021/acs.jmedchem.3c00812

94. Diastereoselective C–H Functionalizations
Cizikovs, A.; Basens, E. E.; Zagorska, P. A.; Grigorjeva, L. In Comprehensive Chirality, 2nd edition (Ed. J. R. Cossy). 2023, Accepted for publication Elsevier Inc. DOI:10.1016/B978-0-32-390644-9.00101-3
93. Phenylglycinol Derived Chemistry
Lidumniece, E.; Bolsakova, J.; Grigorjeva, L. In Comprehensive Chirality, 2nd edition (Ed. J. R. Cossy). 2023, Accepted for publication Elsevier Inc. DOI:10.1016/B978-0-32-390644-9.00100-1
92. May 1,2-Dithiolane-4-carboxylic Acid and Its Derivatives Serve as a Specific Thioredoxin Reductase 1 Inhibitor?
Nikitjuka, A.; Krims-Davis, K.; Kanepe-Lapsa, I.; Ozola, M.; Žalubovskis, R. Molecules, 2023, 28, 6647. DOI:10.3390/molecules28186647
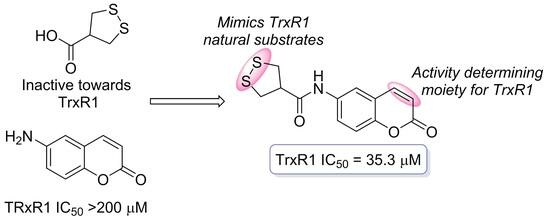
91. Asparagusic Acid – A Unique Approach toward Effective Cellular Uptake of Therapeutics: Application, Biological Targets, and Chemical Properties
Nikitjuka, A.; Žalubovskis, R. ChemMedChem, 2023, 18, e202300143. DOI:10.1002/cmdc.202300143

90. Assembling the Methanoindene Cage of Phragmalin-Type Natural Products
Becica, J.; Rāciņš, O.; Ivanova, M.; Jirgensons, A. J. Org. Chem., 2023, 88, 10306–10309. DOI:10.1021/acs.joc.3c00952

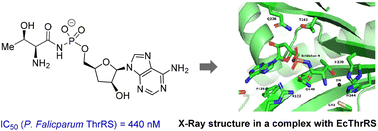
89. Synthesis and evaluation of an agrocin 84 toxic moiety (TM84) analogue as a malarial threonyl tRNA synthetase inhibitor
Rodriguez Buitrago, J. A.; Leitis, G.; Kaņepe-Lapsa, I.; , A.; Parisini, E.; Jirgensons, A. Org. Biomol. Chem., 2023, 21, 5433-5439. DOI:10.1039/D3OB00670K
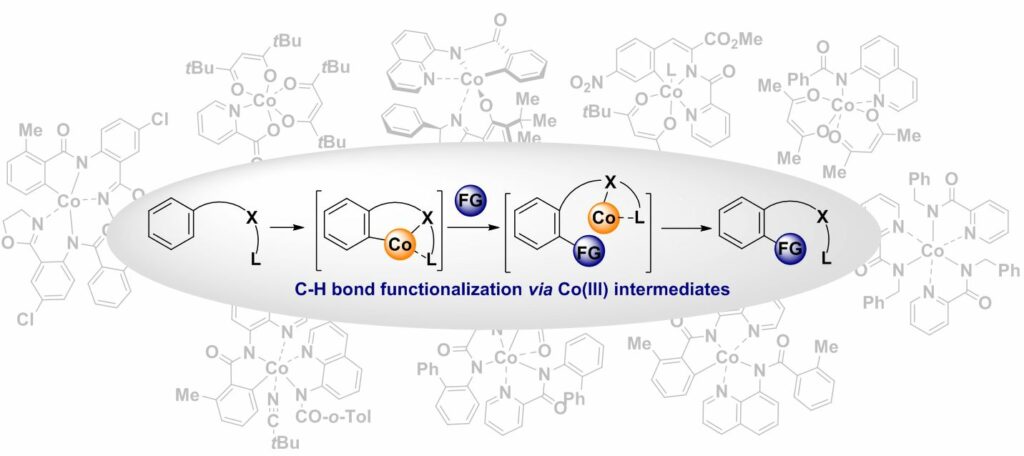
88. Co(III) Intermediates in Cobalt-Catalyzed, Bidentate Chelation Assisted C(sp2)-H Functionalizations
Cizikovs, A.; Grigorjeva, L. Inorganics, 2023, 11(5), 194. DOI:10.3390/inorganics11050194
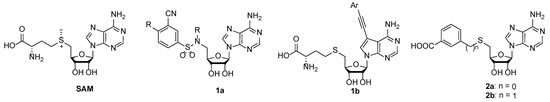
88. 3-(Adenosylthio)benzoic Acid Derivatives as SARS-CoV-2 Nsp14 Methyltransferase Inhibitorswman
Bobileva, O.; Bobrovs, R.; Sirma, E. E.; Kanepe, I.; Bula, A. L.; Patetko, L.; Ramata-Stunda, A.; Grinberga, S.; Jirgensons, A.; Jaudzems, K. Molecules, 2023, 28(2), 768. DOI:10.3390/molecules28020768
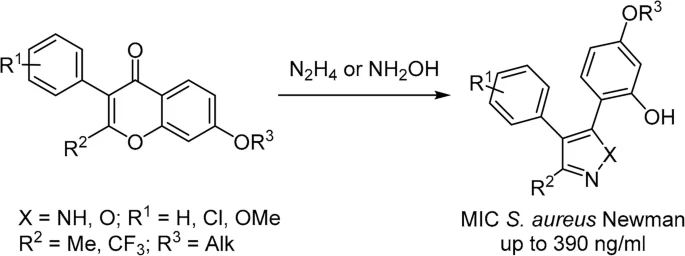
87. Synthesis and SAR of phenylazoles, active against Staphylococcus aureus Newman
Solomin, V. V.; Ciruelos, B. F.; Velikova, N.; Wells, J.; Albanese, M.; Adhav, A.; Jirgensons, A. Chem. Heterocycl. Compd., 2022, 58, 737-748. DOI:10.1007/s10593-023-03151-9
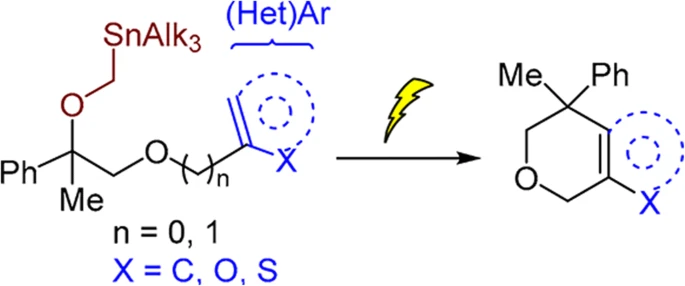
86. Intramolecular Friedel–Crafts alkylation by electrochemical carbenium ion generation
Lielpētere, A.; Šilaks, A.; Jirgensons, A. Chem. Heterocycl. Compd., 2022, 58, 732-736. DOI:10.1007/s10593-023-03150-w
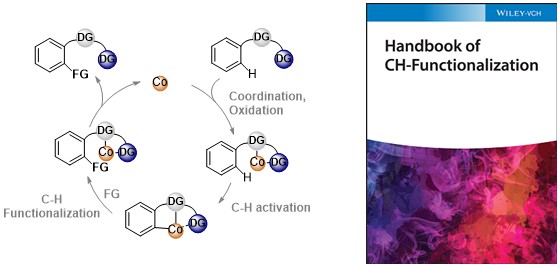
85. Mechanistic Studies on Cobalt-Catalyzed, Bidentate Chelation-Assisted C-H Bond Functionalization
Lukasevics, L.; Grigorjeva, L. In Handbook of C–H Functionalization, Maiti, D. Ed., Wiley, (2022), In Press. DOI:10.1002/9783527834242.chf0236
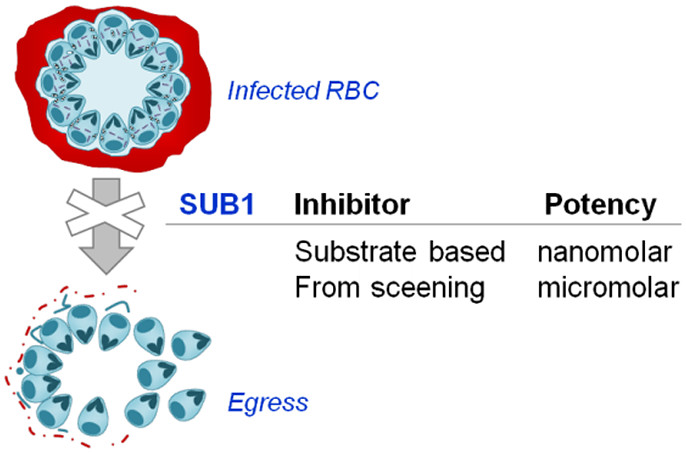
84. Subtilisin-like Serine Protease 1 (SUB1) as an Emerging Antimalarial Drug Target: Current Achievements in Inhibitor Discovery
Lidumniece, E.; Withers-Martinez, C.; Hackett, F.; Blackman, M. J.; Jirgensons, A. J. Med. Chem., 2022, 65, 12535-12545. DOI:10.1021/acs.jmedchem.2c01093
83. Cobalt-catalyzed C(sp2)–H bond imination of phenylalanine derivatives
Lukasevics, L.; Cizikovs, A.; Grigorjeva, L. Chem. Commun., 2022, 58, 9754-9757. DOI:10.1039/D2CC02334B
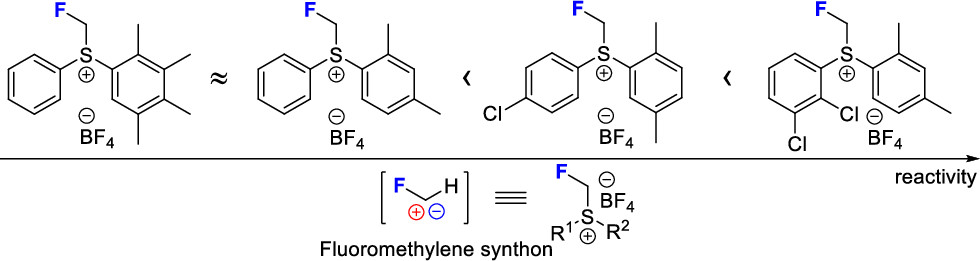
82. Iron-Catalyzed Fluoromethylene Transfer from a Sulfonium Reagent
Sperga, A.; Zacs, D.; Veliks, J. Org. Lett., 2022, 24, 4474–4478. DOI:10.1021/acs.orglett.2c01757

81. Functionalization of Tetrazoles Bearing the Electrochemically Cleavable 1N-(6-Methylpyridyl-2-methyl) Protecting Group
Grammatoglou, K.; Dārziņa, M.; Jirgensons, A. ACS Omega, 2022, 7, 18103–18109. DOI:10.1021/acsomega.2c01633

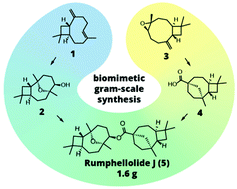
80. Convergent biomimetic semisynthesis of disesquiterpenoid rumphellolide J
Stakanovs, G.; Belyakov, S.; Jirgensons, A.; Rasina, D. Org. Biomol. Chem., 2022, 20, 2455-246. DOI:10.1039/D2OB00238H
79. Localising individual atoms of tryptophan side chains in the metallo-β-lactamase IMP-1 by pseudocontact shifts from paramagnetic lanthanoid tags at multiple sites
Orton, H. W.; Herath, I. D.; Maleckis, A.; Jabar, S.; Szabo, M.; Graham, B.; Breen, C.; Topping, L.; Butler, S. J.; Otting, G. Magn. Reson., 2022, 3, 1-13. DOI:10.5194/mr-3-1-2022
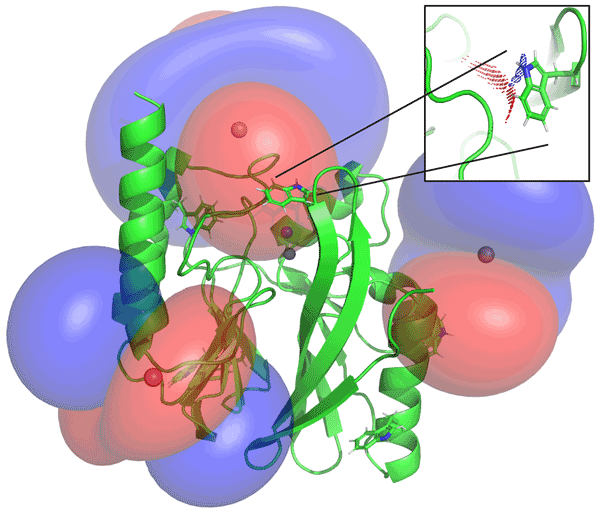
78. Exploring Aspartic Protease Inhibitor Binding to Design Selective Antimalarials
Bobrovs, R.; Basens, E. E.; Drunka, L.; Kanepe, I.; Matisone, S.; Velins, K. K.; Andrianov, V.; Leitis, G.; Zelencova-Gopejenko, D.; Rasina, D.; Jirgensons, A.; Jaudzems, K. J. Chem. Inf. Model., 2022, 62, 3263–3273. DOI:10.1021/acs.jcim.2c00422

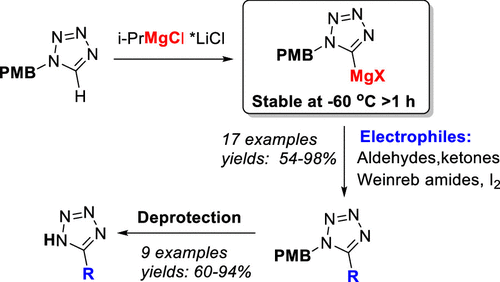
77. Functionalization of 1N-Protected Tetrazoles by Deprotonation with the Turbo Grignard Reagent
Grammatoglou, K.; Jirgensons, A. J. Org. Chem., 2022, 87, 3810-3816. DOI:10.1021/acs.joc.1c02926
76. Synthesis of fluorinated leucines, valines and alanines for use in protein NMR
Maleckis, A.; Abdelkader, E. H.; Herath, I. D.; Otting, G. Org. Biomol. Chem., 2022, 20, 2424-2432. DOI:10.1039/D2OB00145D

75. Discovery of sars-cov-2 nsp14 and nsp16 methyltransferase inhibitors by high-throughput virtual screening
Babrovs, R.; Kanepe, I.; Narvaiss, N.; Patetko, L.; Kalnins, G.; Sisovs, M.; Bula, A. L.; Grinberga, S.; Boroduskis, M.; Ramata-Stunda, A.; Rostoks, N.; Jirgensons, A. Pharmaceuticals, 2021, 14, 1243. DOI:10.3390/ph14121243
74. C–H bond functionalization by high-valent cobalt catalysis: current progress, challenges and future perspectives
Lukasevics, L.; Cizikovs, A.; Grigorjeva, L. Chem. Commun., 2021, 57, 10827-10841. DOI:10.1039/D1CC04382J
73. Torii-Type Electrosynthesis of α,β-Unsaturated Esters from Furfurylated Ethylene Glycols and Amino Alcohols
Darzina, M.; Lielpetere, A.; Jirgensons, A. Eur. J. Org. Chem., 2021, 2021, 4224. DOI:10.1002/ejoc.202100605

72. Synthetic Applications of Monofluoromethylsulfonium Salts
Melngaile, R.; Veliks, J. Synthesis 2021, 53, 4549-4558. DOI:10.1055/a-1548-8240

71. Cobalt-Catalyzed C–H Bond Functionalization Using Traceless Directing Group
Cizikovs, A.; Lukasevics, L.; Grigorjeva, L. Tetrahedron 2021, 93, 132307. DOI:10.1016/j.tet.2021.132307

70. Cobalt-Catalyzed Picolinamide-Directed Synthesis of Heterocycles
Lukasevics, L.; Grigorjeva, L. Targets Heterocycl. Syst. (book series) 2021, 25, 144-161. DOI:10.17374/targets.2022.25.144
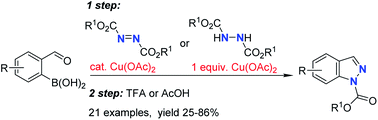
69. Synthesis of Indazoles From 2-Formylphenylboronic Acids
Solomin, V. V.; Seins, A.; Jirgensons, A. RSC Adv. 2021, 11, 22710-22714. DOI:10.1039/D1RA04056A
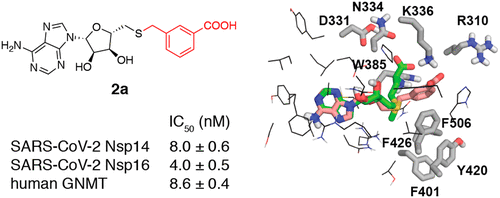
68. Potent SARS-CoV-2 mRNA Cap Methyltransferase Inhibitors by Bioisosteric Replacement of Methionine in SAM Cosubstrate
Bobiļeva, O.; Bobrovs, R.; Kaņepe, I.; Patetko, L.; Kalniņš, G.; Šišovs, M.; Bula, A. L.; Grīnberga, S.; Borodušķis, M.; Ramata-Stunda, A.; Rostoks, N.; Jirgensons, A.; Tārs, K.; Jaudzems, K. ACS Med. Chem. Lett. 2021, 12, 1102-1107. DOI:10.1021/acsmedchemlett.1c00140
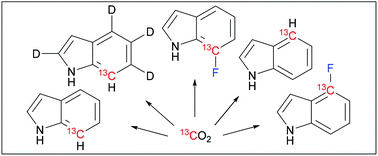
67. Synthesis of 13C/19F/2H labeled indoles for use as tryptophan precursors for protein NMR spectroscopy
Maleckis, A.; Herath, I. D.; Otting, G. Org. Biomol. Chem., 2021, 19, 5133-5147. DOI:10.1039/D1OB00611H
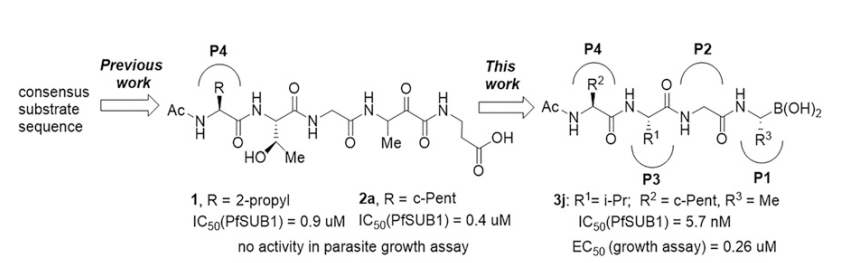
66. Peptidic boronic acids are potent cell-permeable inhibitors of the malaria parasite egress serine protease SUB1
Lidumniece, E.; Withers-Martinez, C.; Hackett, F.; Collins, C. R.; Perrin, A. J.; Koussis, K.; Bisson, C.; Blackman, M. J.; Jirgensons, A. Proc. Natl. Acad. Sci. U.S.A., 2021, 118, e2022696118. DOI:10.1073/pnas.2022696118
65. Sulfonium, (Fluoromethyl)phenyl(2,3,4,5-tetramethylphenyl)-, Tetrafluoroborate(1-) (1:1)
Melngaile, R.; Veliks, J. G. Encycl. Reagents Org. Synth., 2021, pp 1-4. DOI:10.1002/047084289X.rn02379

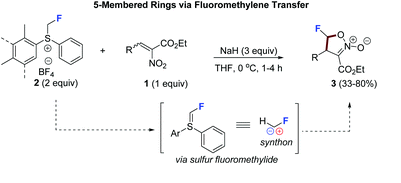
64. Cobalt-Catalyzed C(sp2)–H Carbonylation of Amino Acids Using Picolinamide as a Traceless Directing Group
Lukasevics, L.; Cizikovs, A.; Grigorjeva, L. Org. Lett., 2021, 23, 2748–2753. DOI:10.1021/acs.orglett.1c00660
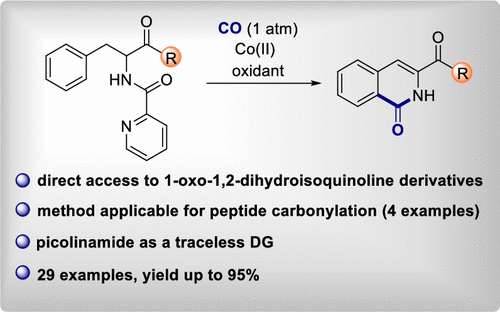
63. Monofluorinated 5-membered rings via fluoromethylene transfer: synthesis of monofluorinated isoxazoline N-oxides
Sperga, A.; Kazia, A.; Veliks, J. Org. Biomol. Chem., 2021, 19, 2688-2691. DOI:10.1039/D1OB00270H
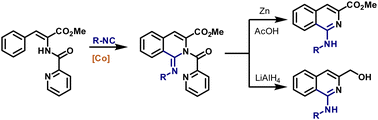
62. Synthesis of 1,2-Dihydroisoquinoline-1-Carboxylates Under Cobalt Catalysis
Zagorska, P. A.; Grigorjeva, L.; Bolsakova, J. Chem. Heterocycl. Compd., 2021, 57(2), 159-165. DOI:10.1007/s10593-021-02888-5
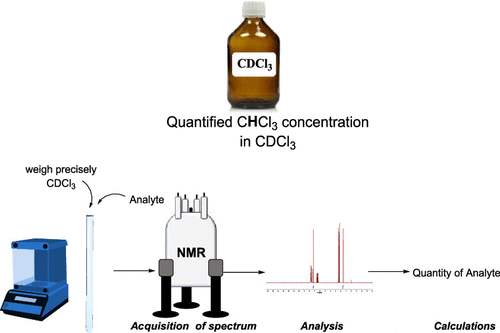
61. Residual Solvent Signal of CDCl3 as a qNMR Internal Standard for Application in Organic Chemistry Laboratory
Muhamadejev, R.; Melngaile, R.; Paegle, P.; Zibarte, I.; Petrova, M.; Jaudzems, K.; Veliks, J. J. Org. Chem., 2021, 86, 3890-3896. DOI:10.1021/acs.joc.0c02744
60. Optimized Monofluoromethylsulfonium Reagents for Fluoromethylene-Transfer Chemistry
Sperga, A.; Melngaile, R.; Kazia, A.; Belyakov, S.; Veliks, J. J. Org. Chem., 2021, 86, 3196–3212. DOI:10.1021/acs.joc.0c02561
59. Cell‐Free Synthesis of Selenoproteins in High Yield and Purity for Selective Protein Tagging
Welegedara, A. P.; Maleckis, A.; Bandara, R.; Mahawaththa, M. C.; Herath, I. D.; Tan, Y. J.; Giannoulis, A.; Goldfarb, D.; Otting, G.; Huber, T. ChemBioChem, 2021, 22, 1480-1486. DOI:10.1002/cbic.202000785

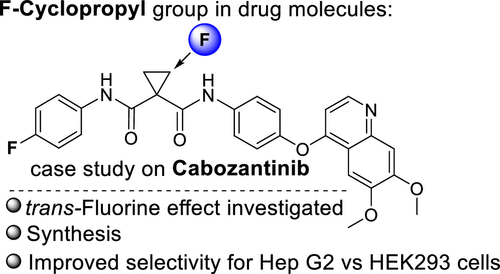
58. trans-Fluorine Effect in Cyclopropane: Diastereoselective Synthesis of Fluorocyclopropyl Cabozantinib Analogs
Veliks, J.; Videja, M.; Kinens, A.; Bobrovs, R.; Priede, M., Kuka, J. ACS Med. Chem. Lett., 2020, 11(11), 2146-2150. DOI:10.1021/acsmedchemlett.0c00220
57. Cobalt-Catalyzed Carbonylation of The C–H Bond
Lukasvics, L.; Grigorjeva, L., Org. Biomol. Chem., 2020, 18, 7460-7466. DOI:10.1039/D0OB01633K
56. Friedel–Crafts Alkylation with Carbenium Ions Generated by Electrochemical Oxidation of Stannylmethyl Ethers
Lielpetere, A.; Jirgensons, A., Eur. J. Org. Chem., 2020, 29, 4510-4516. DOI:10.1002/ejoc.202000568

55. 2-Aminoquinazolines by Chan–Evans–Lam Coupling of Guanidines with (2-Formylphenyl)boronic Acids
Solomin, V. V.; Seins, A.; Jirgensons, A., Synlett, 2020, 31, 1507-1510. DOI:10.1055/s-0040-1707080

54. Synthesis of 2-Aminopyridopyrimidinones and Their Plasmepsin I, II, IV Inhibition Potency
Rasina, D.; Stakanovs, G.; Kanepe-Lapsa, I.; Bobrovs, R.; Jirgensons, A., Chem. Heterocycl. Compd., 2020, 56, 786-792. DOI:10.1007/s10593-020-02731-3
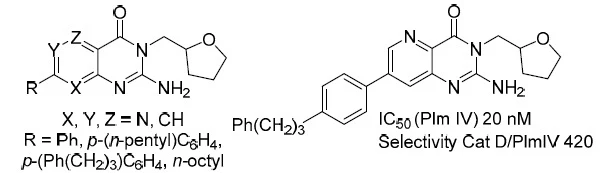
53. A Concise Bioinspired Semisynthesis of Rumphellaones A–C and Their C-8 Epimers from β-Caryophyllene
Stakanovs, G.; Mishnev, A.; Rasina, D.; Jirgensons, A., J. Nat. Prod. 2020, 83, 6, 2004-2009. DOI:10.1021/acs.jnatprod.0c00403
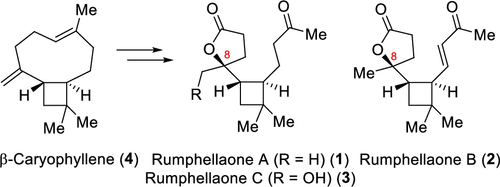
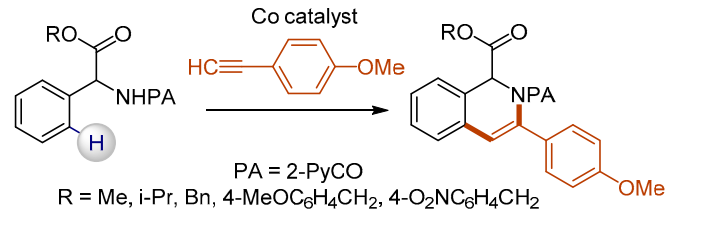
52. Synthesis of 3-Hydroxymethyl Isoindolinones via Cobalt-Catalyzed C(sp2)–H Carbonylation of Phenylglycinol Derivatives
Lukasevics, L.; Cizikovs, A.; Grigorjeva, L., Org. Lett. 2020, 22, 2720-2723. DOI:10.1021/acs.orglett.0c00672
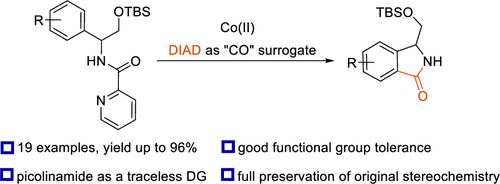
51. Cobalt-Catalyzed, Directed C–H Functionalization/Annulation of Phenylglycinol Derivatives with Alkynes
Bolsakova, J.; Lukasevics, L.; Grigorjeva, L., J. Org. Chem. 2020, 85, 4482-4499. DOI:10.1021/acs.joc.0c00207
50. Johnson–Corey–Chaykovsky Fluorocyclopropanation of Double Activated Alkenes: Scope and Limitations
Kazia, A.; Melngaile, R.; Mishnev, A.; Veliks, J. Org. Biomol. Chem. 2020, 18, 1384-1388. DOI:10.1039/C9OB02712B
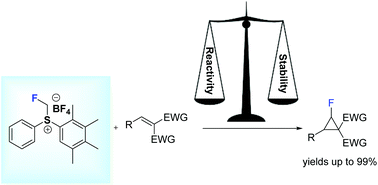
49. Diastereoselective Monofluorocyclopropanation Using Fluoromethylsulfonium Salts
Melngaile, R.; Sperga, A.; Baldridge, K. K.; Veliks, J. Org. Lett. 2019, 21,7174-7178. DOI:10.1021/acs.orglett.9b02867

48. Exploiting Structural Dynamics to Design Open-Flap Inhibitors of Malarial Aspartic Proteases
Bobrovs, R.; Jaudzems, K.; Jirgensons, A. J. Med. Chem., 2019, 62, 20, 8931-8950. DOI:10.1021/acs.jmedchem.9b00184

47. Fluoromethylene Transfer from Diarylfluoromethylsulfonium Salts: Synthesis of Fluorinated Epoxides
Veliks, J.; Kazia, A. Chem. Eur. J., 2019, 25, 3786 –3789. DOI:10.1002/chem.201900349
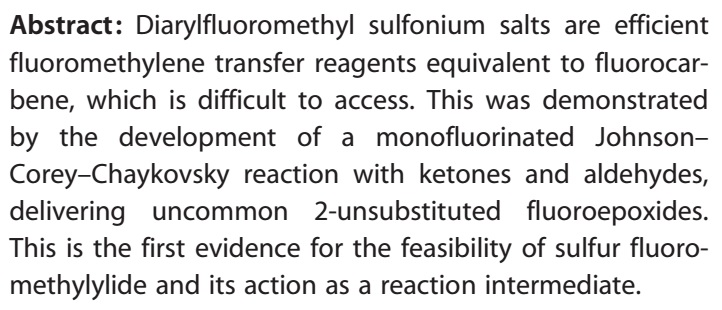
46. A Novel Collaborative Approach to Facilitate Chemical Biology
Brennecke, P.; Rasina, D.; Aubi, O.; Herzog, K.; Landskron, J.; Cautain, B.; Vicente, F.; Quintana, J.; Mestres, J.; Stechmann, B.; Ellinger, B.; Brea, J; Kolanowski, J. L.; Pilarski, R.; Orzaez, M.; Pineda-Lucena, A.; Laraia, L.; Nami, F.; Zielenkiewicz, P.; Paruch, K.; Hansen, E.; Von Kries, J. P.; Neuenschwander, M.; Specker, E.; Bartunek, P.; Simova, S.; Leśnikowski, Z.; Krauss, S.; Lehtiö, L.; Bilitewski, U.; Brönstrup, M.; Taskén, K.; Jirgensons, A.; Lickert, H.; Clausen, M. H.; Andersen, J. H.; Vicent, M. J.; Genilloud, O.; Martinez, A.; Nazaré, M.; Fecke, W.; Gribbon, P.
SLAS Discov., 2019, 24, 398–413. DOI:10.1177/2472555218816276

45. Amination of Carbenium Ions Generated by Directed Protonolysis of Cyclopropane
Skvorcova, M.; Lukasevics, T. L.; Jirgensons, A. J. Org. Chem., 2019, 84, 3780-3792. DOI:10.1021/acs.joc.8b02576

44. Peptidomimetic Plasmepsin Inhibitors with Potent Anti-Malarial Activity and Selectivity Against Cathepsin D
Zogota, R.; Kinena, L.; Withers-Martinez, C.; Blackman, M. J.; Bobrovs, R.; Pantelejevs, T.; Kanepe-Lapsa, I.; Ozola, V.; Jaudzems, K.; Suna, E.; Jirgensons, A. Eur. J. Med. Chem., 2019, 163, 344-352. DOI:10.1016/j.ejmech.2018.11.068

43. Refining the Structure−Activity Relationships of 2-Phenylcyclopropane Carboxylic Acids as Inhibitors of O-Acetylserine Sulfhydrylase Isoforms
Magalhães, J.; Franko, N.; Annunziato, G.; Pieroni, M.; Benoni, R.; Nikitjuka, A.; Mozzarelli, A.; Bettati, S.; Karawajczyk, A.; Jirgensons, A.; Campanini, B.; Costantino, G. J. Enzyme Inhib. Med. Chem., 2019, 34, 31-43. DOI:10.1080/14756366.2018.1518959
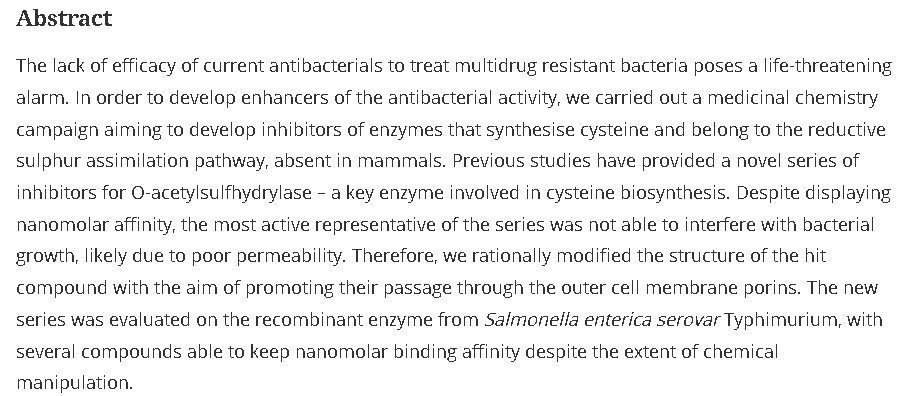
42. Azole-Based Non-Peptidomimetic Plasmepsin Inhibitors
Kinena, L.; Leitis, G.; Kanepe-Lapsa, I.; Bobrovs, R.; Jaudzems, K.; Ozola, V.; Suna, E., Jirgensons, A. Arch. Pharm. Chem. Life Sci., 2018, 351, 1800151. DOI:10.1002/ardp.201800151
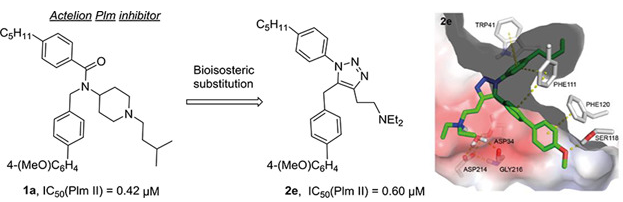
41. 2-Aminoquinazolin-4(3H)-one Based Plasmepsin Inhibitors with Improved Hydrophilicity and Selectivity
Rasina, D.; Stakanovs, G.; Borysov, O.V.; Pantelejevs, T.; Bobrovs, R.; Kanepe-Lapsa, I.; Tars, K.; Jaudzems, K.; Jirgensons, A. Biorg. Med. Chem., 2018, 26(9), 2488-2500. DOI:10.1016/j.bmc.2018.04.012

40. N-Leucinyl Benzenesulfonamides as Structurally Simplified Leucyl-tRNA Synthetase Inhibitors
Charlton, M.H.; Aleksis, R.; Saint-Leger, A.; Gupta, A; Loza, E.; , Ribas De Pouplana, L.; Kaula, I.; Gustina, D.; Madre, M.; Lola, D; Jaudzems, K.; Edmund, G.; Randall, C.P.; Kime, L.; O’Neill, A.J.; Goessens, W.; Jirgensons, A.; Finn, P.W. ACS Med. Chem. Lett. 2018, 9(2), 84-88. DOI:10.1021/acsmedchemlett.7b00374

39. Carbenium Ion Formation by Fragmentation of Electrochemically Generated Oxonium Ions
Lielpetere, A.; Jirgensons, A. Org. Biomol. Chem., 2018, 16, 5094-5096. DOI:10.1039/c8ob01339j

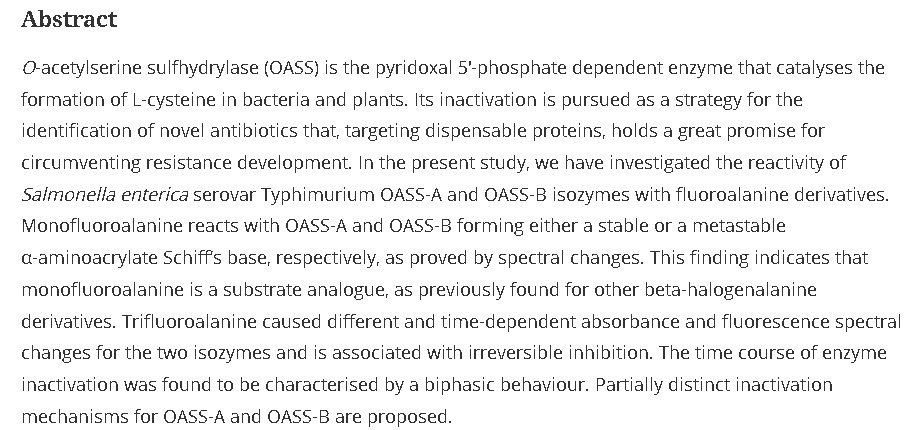
38. Inhibition of O-Acetylserine Sulfhydrylase by Fluoroalanine Derivatives
Franko, N.; Grammatoglou, K.; Campanini, B.; Costantino, G.; Jirgensons, A.; Mozzarelli, A. J. Enzyme Inhib. Med. Chem., 2018, 33, 1343-1351. DOI:10.1080/14756366.2018.1504040
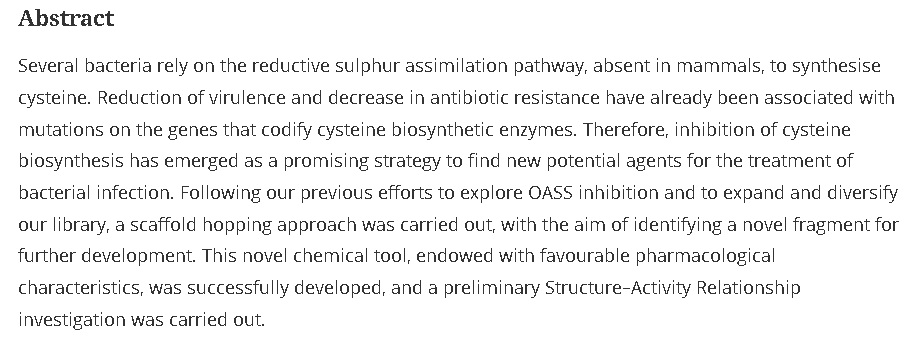
37. Discovery of Novel Fragments Inhibiting O-Acetylserine Sulphhydrylase by Combining Scaffold Hopping and Ligand–Based Drug Design
Magalhães, J.; Franko, N.; Annunziato, G.; Welch, M.; Dolan, S. K.; Bruno, A.; Mozzarelli, A.; Armao, S.; Jirgensons, A.; Pieroni, M.; Costantino, G.; Campanini, B. J. Enzyme Inhib. Med. Chem., 2018, 33, 1444-1452. DOI:10.1080/14756366.2018.1512596
36. The Ritter Reaction for the Synthesis of Heterocycles (Minireview)
Bolsakova, J.; Jirgensons, A. Chem. Heterocycl. Compd., 2017, 53(11), 1167-1177. DOI:10.1007/s10593-018-2189-y
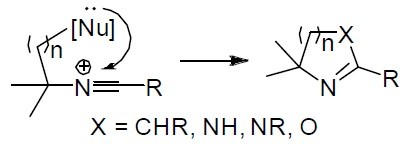
35. 1-Amino-1-hydroxymethylcyclobutane Derivatives via Intramolecular Amination of Nonclassical Cyclopropylmethyl Cation
Skvorcova, M., Grigorjeva, L., Jirgensons, A. Chem. Heterocycl. Compd., 2017, 53(9), 989-996. DOI:10.1007/s10593-017-2162-1
34. Amide-Group-Directed Protonolysis of Cyclopropane: An Approach to 2,2-Disubstituted Pyrrolidines
Skvorcova, M.; Jirgensons, A. Org. Lett., 2017, 19, 2478-2481. DOI:10.1021/acs.orglett.7b00584

33. N-Sulfonylcarboxamide as an Oxidizing Directing Group for Ruthenium-Catalyzed C–H Activation/Annulation
Petrova, E.; Rasina, D.; Jirgensons, A. Eur. J. Org. Chem., 2017, 1773-1779. DOI:10.1002/ejoc.201601582

32. Intramolecular Cyclopropylmethylation via Non-Classical Carbocations
Skvorcova, M.; Jirgensons, A. Org. Biomol. Chem., 2017, 15, 6909-6912. DOI:10.1039/C7OB01721A
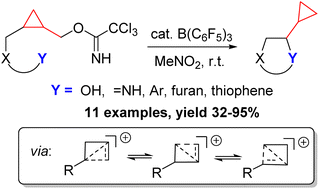
31. C-Quaternary Alkynyl Glycinols via the Ritter Reaction of Cobalt Complexed Alkynyl Glycols
Grammatoglou, K.; Bolsakova, J.; Jirgensons, A. RSC Adv., 2017, 7, 27530-27537. DOI:10.1039/C7RA03965D

30. Allylic Amination via Acid Catalyzed Leaving Group Activation
Skvorcova, M.; Jirgensons, A. Curr. Green Chem., 2016, 3(2), 145-159. DOI:10.2174/2213346103666160905101423
29. Evaluation of the Characteristics of Leucyl-tRNA Synthetase (LeuRS) Inhibitor AN3365 in Combination with Different Antibiotic Classes
Monteferrante, C.G.; Jirgensons, A.; Varik, V. et al. Eur. J. Clin. Microbiol. Infect. Dis., 2016, 35, 1857-1864. DOI:10.1007/s10096-016-2738-1
28. Targeting Multiple Aminoacyl-tRNA Synthetases Overcomes the Resistance Liabilities Associated with Antibacterial Inhibitors Acting on a Single Such Enzyme
Randall, C.; Rasina, D.; Jirgensons, A.; O’Neill, A. Antimicrob. Agents Chemother., 2016, 60(10) 6359-6361. DOI:10.1128/AAC.00674-16

27. Crystal Structure of Plasmodium Falciparum Proplasmepsin IV: the Plasticity of Proplasmepsins
Recacha, R.; Jaudzems, K.; Akopjana, I.; Jirgensons, A.; Tars, K.
Acta Cryst., 2016, F72, 659-666. DOI:10.1107/S2053230X16011663
26. A Polymorphism in LeuS Confers Reduced Susceptibility to GSK2251052 in a Clinical Isolate of Staphylococcus Aureus
Gupta, A.; Monteferrante, C.; Rasina, D.; Leitis, G.; Randall, C. P.; Tomlinson, J. H.; Jirgensons, A.; Goessens, W.H.F.; Hays, J.P.; O’Neill, A.J.
Antimicrob. Agents Chemother., 2016, 60(5), 3219-3221. DOI:10.1128/AAC.02940-15

25. Fragment-Based Discovery of 2-Aminoquinazolin-4(3H)-ones as Novel Class Nonpeptidomimetic Inhibitors of the Plasmepsins I, II, and IV
Rasina, D.; Otikovs, M.; Leitans, J; . Recacha, R.; Borysov, O.V.; Kanepe-Lapsa, I.; Domraceva, I.; Pantelejevs, T.; Tars, K.; Blackman, M.J.; Jaudzems, K.; Jirgensons, A. J. Med. Chem., 2016, 59, 374–387. DOI:10.1021/acs.jmedchem.5b01558

24. Synthesis of α‐Ethynyl Glycines (Microreview)
Bolsakova, J.; Jirgensons, A. Eur. J. Org. Chem., 2016, 27, 4591-4602. DOI:10.1002/ejoc.201600253
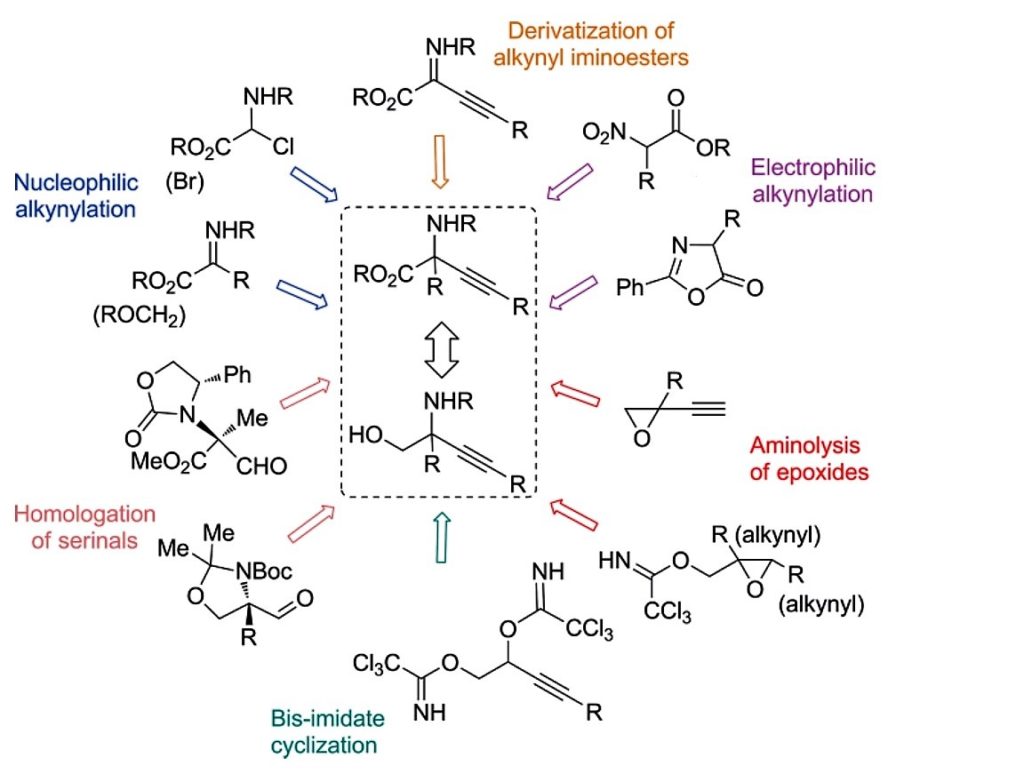
23. Synthesis of Alkynyl‐Glycinols by Lewis Acid Catalyzed Propargylic Substitution of Bis‐Imidates
Sirotkina, J.; Grigorjeva, L.; Jirgensons, A. Eur. J. Org. Chem. 2015, 6900-6908. DOI: 10.1002/ejoc.201500937

22. Synthesis and Biological Evaluation of Aziridin-1-yl Oxime-based Vorinostat Analogs as Anticancer Agents
Nikitjuka, A.; Shestakova, I.; Romanchikova, N.; Jirgensons A. Chem. Heterocycl. Compd., 2015, 51(7), 647-657. DOI:10.1007/s10593-015-1752-z

21. Tetrahydro-1,3-oxazepines via Intramolecular Amination of Cyclopropylmethyl Cation
Skvorcova, M.; Grigorjeva, L.; Jirgensons, A. Org. Lett., 2015, 17, 2902–2904. DOI:10.1021/acs.orglett.5b01014

20. 2-Vinyl Threoninol Derivatives via Acid-Catalyzed Allylic Substitution of Bisimidates
Kumar, V.; Klimovica, K.; Rasina, D. Jirgensons A. J. Org. Chem., 2015, 80, 5934–5943. DOI:10.1021/acs.joc.5b00529

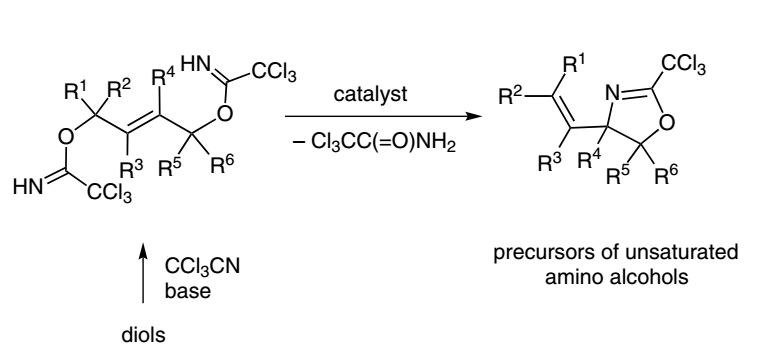
19. Unsaturated syn– and anti-1,2-Amino Alcohols by Cyclization of Allylic Bis-trichloroacetimidates. Stereoselectivity Dependence on Substrate Configuration
Grigorjeva, L; Kinens, A.; Jirgensons, A. J. Org. Chem., 2015, 80, 920–927. DOI:10.1021/jo502404y

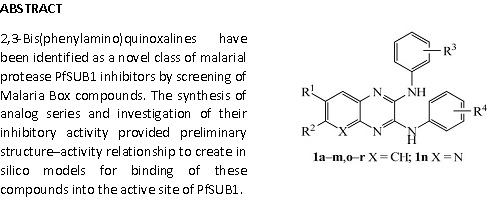
18. Quinoxaline Based Inhibitors of Malarial Protease PfSUB
Kher, S.S.; Penzo, M.; Fulle, S.; Ebejer, J.P.; Finn, P.W. ; Blackman, M.J.; Jirgensons, A. Chem. Heterocycl. Compd., 2015, 50, 1457-1463. DOI:10.1007/s10593-014-1610-4
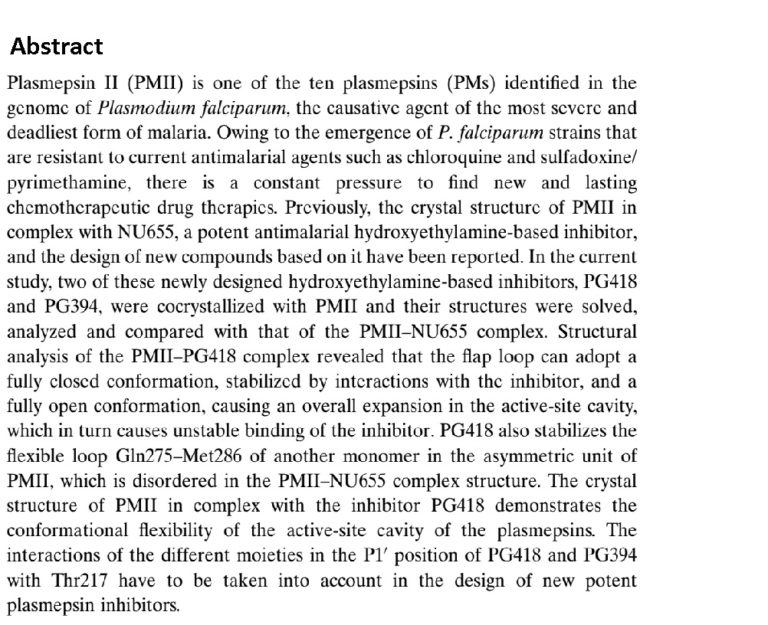
17. Structures of Plasmepsin II from Plasmodium Falciparum in Complex with Two Hydroxyethylamine-Based Inhibitors
Recacha R.; Leitans J.; Akopjana I.; Aprupe L.; Trapencieris P.; Jaudzems K.; Jirgensons A.; Tars K. Acta Cryst. 2015. F71, 1531-1539. DOI:10.1107/S2053230X15022049
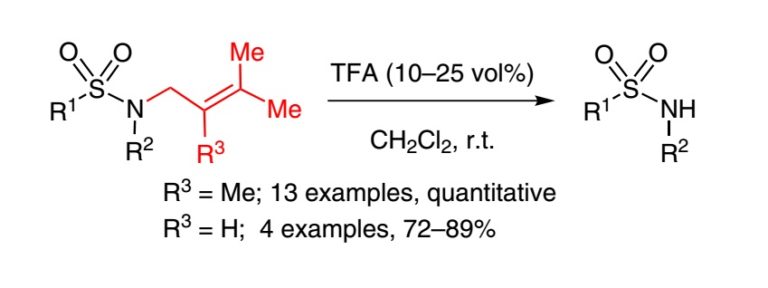
16. Methylprenyl and Prenyl Protection for Sulfonamides
Nikitjuka, A.; Nekrasova, A.; Jirgensons A. Synlett, 2015, 26, 183-186. DOI:10.1055/s-0034-1379428
15. Substrate Derived Peptidic α-Ketoamides as Inhibitors of the Malarial Protease PfSUB1
Kher, S.S.; Penzo, M.; Fulle, S.; Finn, P.W. ; Blackman, M.J.; Jirgensons, A. Bioorg. Med. Chem. Lett., 2014, 24(18), 4486-4489. DOI:10.1016/j.bmcl.2014.07.086

14. Semisynthesis of Libiguin A and Its Analogues by Trans-Lactonization of Phragmalin
Grigorjeva, L.; Liepinsh, E.; Razafimahefa, S.; Yahorau, A.; Yahorava, S.; Rasoanaivo, P.; Jirgensons, A.; Wikberg, J.E.S. J. Org. Chem., 2014, 79(9), 4148–4153. DOI:10.1021/jo500318w

13. Plasmepsin Inhibitory Activity and Structure-Guided Optimization of a Potent Hydroxyethylamine-Based Antimalarial Hit
Jaudzems, K.; Tars, K.; Maurops, G.; Ivdra, N; Otikovs, M. ; Leitans, J.; Kanepe-Lapsa, I.; Domraceva, I.; Mutule, I.; Trapencieris, P.; Blackman, M.J.; Jirgensons, A. ACS Med. Chem. Lett., 2014, 5(4), 373–377. DOI:10.1021/ml4004952

12. Synthesis of β-Amino-α-ketoamides (Review)
Kher, S.S.; Jirgensons, A. Curr. Org. Chem., 2014, 18, 2240-2269. DOI:10.2174/1385272819666140818223225

11. Synthesis, Chemical and Biological Properties of Aziridine-1-carbaldehyde Oximes (Minireview)
Nikitjuka, A.; Jirgensons, A. Chem. Heterocycl. Compd., 2014, 49, 1544-1559. Dedicated to Professor Janis Stradins on the occasion of his 80 anniversary. DOI:10.1007/s10593-014-1407-5
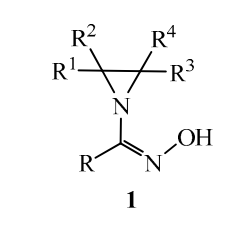
10. Unsaturated Amino Alcohols via Cyclization of Allylic Bistrichloroacetimidates
Jirgensons, A.; Grigorjeva, L.; Maleckis, A.; Klimovica, K. Synlett, 2013, 24(18), 2345-2349. DOI:10.1055/s-0033-1338977
9. Synthesis of 5-Substituted 3-Mercapto-1,2,4-Triazoles via Suzuki–Miyaura Reaction
Katkevica, S.; Salun, P.; Jirgensons, A. Tetrahedron Lett., 2013, 54, 4524–4525. DOI:10.1016/j.tetlet.2013.06.067

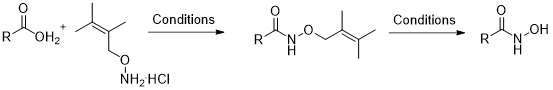
8. Synthesis of Hydroxamic Acids by Using the Acid Labile O-2-Methylprenyl Protecting Group
Nikitjuka, A; Jirgensons, A. Synlett, 2012, 23, 2972-2974. DOI:10.1055/s-0032-1317687
7. Synthesis of Cyclic N-Tosyl-iminocarbonates by Lewis Acid Catalysed Allylic Substitution of Trichloroacetimidates
Grigorjeva, L.; Jirgensons, A. Eur. J. Org. Chem., 2012, 27, 5307–5316. DOI:10.1002/ejoc.201200378

6. Plasmodium Subtilisin-like Protease 1 (SUB1): Insights into the Active-Site Structure, Specificity and Function of a Pan-Malaria Drug Target
Withers-Martinez, C.; Suarez, C.; Fulle, S.; Kher, S.; Penzo, M.; Ebejer, J.-P.; Koussis, K.; Hackett, F.; Jirgensons, A.; Finn, P.; Blackman, M.J. Int. J. Parasitol., 2012, 42, 597-612. DOI:10.1016/j.ijpara.2012.04.005

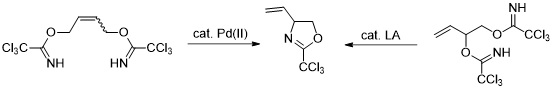
5. Novel Synthesis of 2-Trichloromethyl-4-vinyl-oxazoline and its Derivatization by Ring Cleavage Reactions
Grigorjeva, L.; Maleckis, A.; Klimovica, K.; Skvorcova, M.; Ivdra, N.; Leitis, G.; Jirgensons, A. Chem. Heterocycl. Compd., 2012, 48, 919-924. Dedicated to professor Ivars Kalvinsh on the occasion of his 65th birthday. DOI:10.1007/s10593-012-1077-0
4. C-Quaternary Vinylglycinols by Metal Catalysed Cyclization of Allylic Bis-Trichloroacetimidates
Klimovica, K.; Grigorjeva, L.; Maleckis, A.; Popelis, J.; Jirgensons, A. Synlett, 2011, 2849-2851. DOI:10.1055/s-0031-1289537
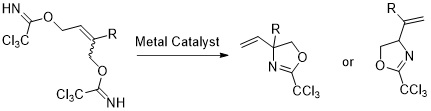
3. Lewis Acid Catalyzed Intramolecular Allylic Substitution of Bis(trichloroacetimidates): A Versatile Approach to Racemic Unsaturated Amino Acids
Grigorjeva, L.; Jirgensons, A. Eur. J. Org. Chem., 2011, 2421–2425. DOI:10.1002/ejoc.201100060
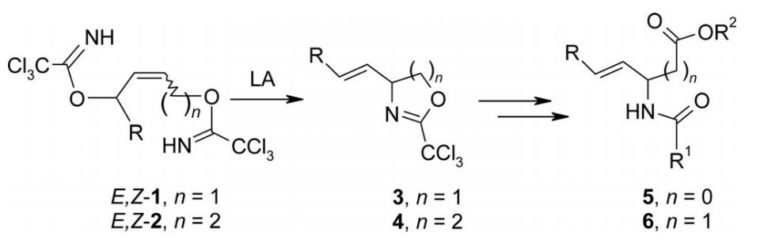
2. Catalytic Enantioselective Synthesis of 4-Vinyl-2-Trichloromethyloxazoline: An Access to Enantioenriched Vinylglycinol Surrogate
Maleckis, A.; Klimovica, K.; Jirgensons, A. J. Org. Chem., 2010, 75, 7897-7900. DOI:10.1021/jo101781y

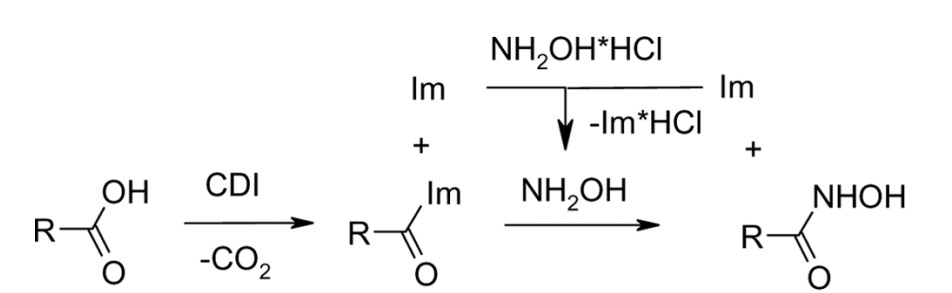
1. Synthesis of Hydroxamic Acids by Activation of Carboxylic Acids with N,N’-Carbonyldiimidazole: Exploring the Efficiency of the Method
Usachova, N.; Leitis, G.; Jirgensons, A.; Kalvinsh I. Synthetic Comm., 2010, 40, 927–935. DOI:10.1080/00397910903026723
Patents
7. 2-Amino-n-(amino-oxo-aryl-lambda6-sulfanylidene)acetamide compounds and their therapeutic use.
Jirgensons A., Finn P. W., Edmund G., Skvorcova M., Veliks J., Charlton M. H., Grigorjeva L. Patent No WO2021123237, A1, 2021.
6. 2-Amino-n-(amino-oxo-aryl-lambda6-sulfanylidene)acetamide compounds and their therapeutic use.
Edmund G., Charlton M. H., Finn P. W., Jirgensons A., Skvorcova M., Veliks J., Grigorjeva L. Patent No EP4077289, A1, 2020.
5. Novel Compounds, Their Manufacture and Uses (II)
Wikberg, J.; Jirgensons A., Liepinsh E. Patent No WO2013110744, A2, 01.08. 2013.
4. Method for Preparing Neramexane or a Salt Thereof
Gold M.R.; Kauss V.; Jirgensons A; Patent No WO2011035924, A1, 31. 03. 2011.
3. Method for Producing Memantine
Gold, M.R.; Jirgensons, A; Huber, F.A.M. Patent No WO 2010/069555, A1, 24. 06. 2010.
2. Enantioselektīva 2-trihlormetil-4-viniloksazolīna iegūšana
Maleckis, A.; Jirgensons, A. LV Patent Application No, P-09-77. 2009.
1. Bicyclosulfonyl Acid (BCSA) Compounds and Their Use as Therapheutic Agents
Finn, P; Robinson, D.; Kham, N.; Jirgensons, A.; Leitis, G.; Kalvinsh I. PCT /GB2008/001683, 16.05.2008.